- Call us!
- +86 531 8887 6213
- cathy@winner-psa.com
The classification of physical adsorption isotherms proposed by the International Union of Pure and Applied Chemistry (IUPAC) (Figure 6):
(1) Type I is characterized by a situation close to saturation after a certain pressure. Chemical adsorption limited to single-layer adsorption belongs to this type and is often called the Langmuer type. This also happens with physical adsorption, which often occurs in the adsorption of microporous adsorbents.
Due to the proximity effect of the pore wall, the adsorption energy can be significantly improved. Within a small range of relative pressure, the micropores are gradually filled. Later, as the relative pressure increases, this micropore adsorption has become saturated. The main geometric parameter characterizing micropore filling is the micropore volume rather than the surface area of the micropore walls. For example, the adsorption of organic vapor on activated carbon.
(2) Type II is the most common adsorption isotherm, which is S-shaped. Adsorbents exhibiting this type of isotherm are powders of non-porous particles. For example, the adsorption of nitrogen on Fe powder at -196oC.
(3)The isotherm of type III is concave. Commonly seen in situations where the adsorption effect is very weak.
(4) Type IV isotherm, its interpretation is similar to that of type II. The difference is that the adsorbent contains a considerable number of mesopores, and the capillary condensation adsorbed in the mesopores appears saturated within a certain relative pressure range. For example, the adsorption of benzene vapor on iron oxide gel or silica gel at room temperature.
(5) The difference between type V and type III is just like the difference between type IV and type II. It is also due to the fact that the adsorbent contains a considerable number of mesopores, resulting in capillary condensation and saturation within a certain relative pressure range.
(6) Type VI, also called ladder isotherm, is common when non-polar adsorbates are adsorbed on non-porous solids with uniform physical and chemical properties. For example, carbon is graphitized above 2700oC and then adsorbs nitrogen, argon, and krypton. This ladder-shaped isotherm is formed by first forming the first two- dimensional ordered molecular layer and then adsorbing the second layer. The adsorption of the second layer is obviously influenced by the first layer and therefore becomes a step shape. The adsorbed molecules also show a ladder shape when they undergo corresponding changes, but there is only one step. When type VI interaction occurs, it takes a long time to reach adsorption equilibrium. An obvious step shape also appears when crystal water is formed.
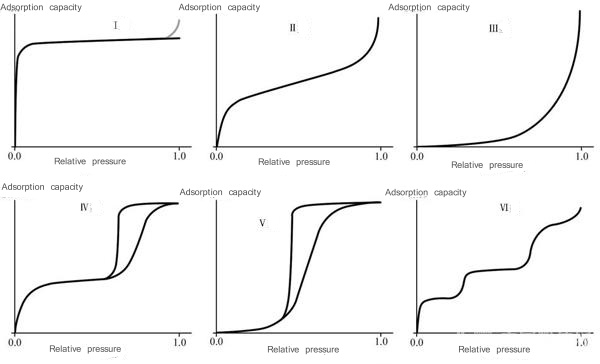
Figure 6 Classification of physical adsorption isotherms proposed by IUPAC
Copyright © Jinan Winner Particle Instrument Stock Co., Ltd. All Rights Reserved | Sitemap
Keywords:
Laser Particle Size Analyzer Spray Particle Size Analyzer Particle Image System Online Particle Size Analyzer Particle Size Analyzer particle size distribution particle size analyzer manufacturer Laser diffraction particle size analyzer particle size malvern particle size analyzer